Next Generation Classification and Therapeutic Stratification of Glioma
Abstract
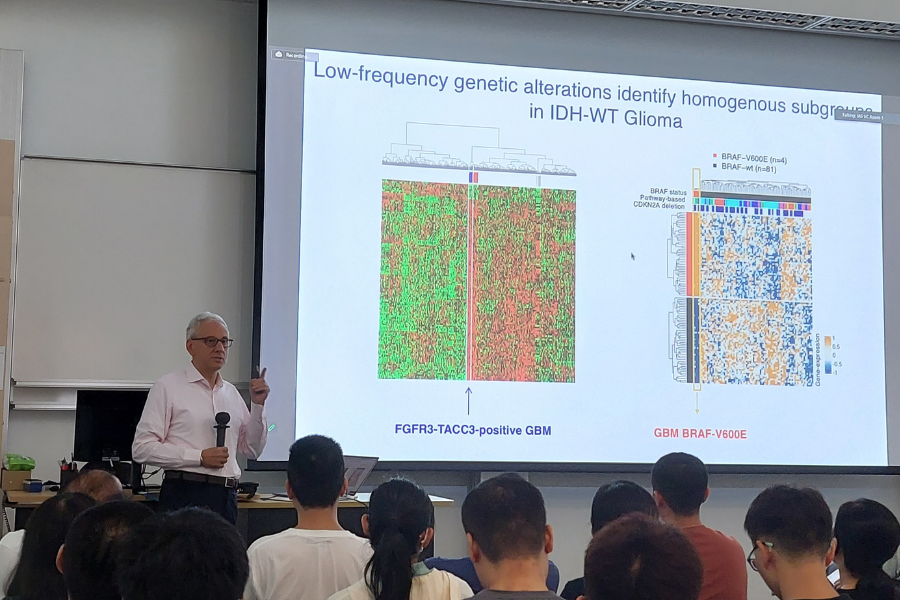
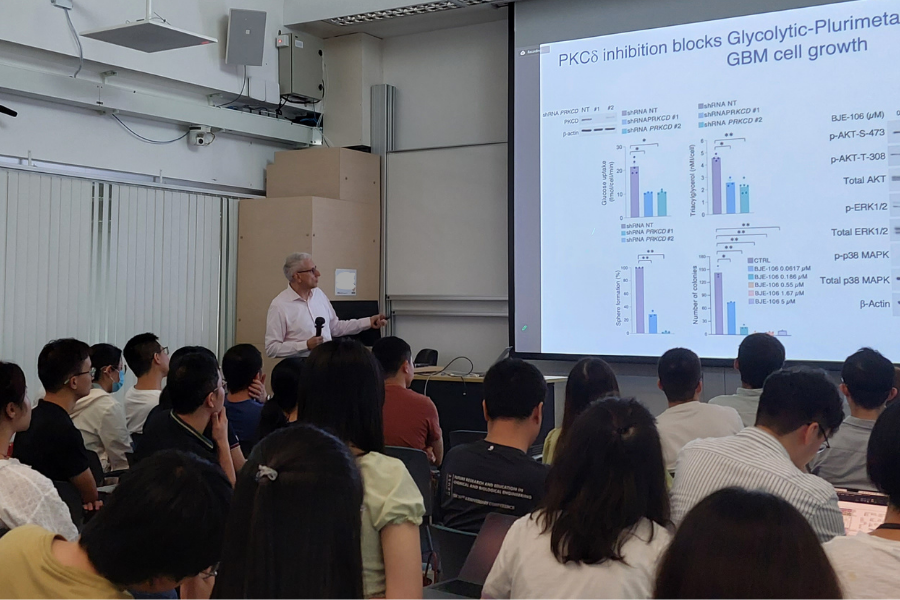
The focus of the speaker’s research program is to identify, stratify, and model key cancer genes from multiple molecular platforms. By focusing on the most significant and unbiased cancer modules, the speaker’s team has undertaken the task of identifying master regulators of cancer initiation and progression in distinct sub-groups of brain tumors. Some of the most notable examples include the ID proteins in cancer stem cells and the Stat3 and C/EBP transcription factors in glioblastomas undergoing progression along the mesenchymal lineage. The team discovered the FGFR3-TACC3 gene fusion in glioblastoma. This translocation has emerged as the single most frequent gene fusion across all human tumors. The team reported that FGFR3-TACC3 fusions trigger tumorigenesis through activation of oxidative phosphorylation. The speaker’s team’s most recent work emphasized the importance of mitochondrial metabolism in tumor growth and enabled the stratification of cancer patients for precision targeting of tumor metabolism. Recently, using an unbiased algorithm based on kinase-phosphorylation site interactions that is applicable to any proteomic dataset, the team identified and experimentally validated two protein kinases (PKCd and DNA-PKcs) as the master kinases that drive two functional subtypes of GBM and are therapeutic targets of other cancer subtypes.
About the Speaker
Prof. Antonio IAVARONE is a professor in the Department of Neurological Surgery at the University of Miami. He is also the Deputy Director of the Sylvester Comprehensive Cancer Center.
Prof. Iavarone seeks to unravel the biologic and genetic alterations driving subgroups of malignant brain tumors and exploit this information for rational therapeutic stratification. His team identified master regulators of cancer initiation and progression in distinct sub-groups of brain tumors. They discovered the first example of oncogenic and tumor addicting gene fusions in glioblastoma (FGFR3-TACC3) and reported that FGFR3-TACC3 fusions trigger tumorigenesis through activation of oxidative phosphorylation. These fusions are among the most frequent chromosomal translocations across all types of human cancer and the FDA approved the targeting of these chromosomal translocations with FGFR inhibitors.
Prof. Iavarone chaired the Pan-Glioma ATLAS-TCGA Working Group and the Neurofibromatosis 1 synodos that united international researchers to formulate guidelines for accurate diagnosis and prognosis of glioma patients. By using all the computational and experimental tools at disposal, they combine innovations in both areas to continue making transformative mechanistic discoveries and provide personalized therapeutics to increasing number of patients with deadly tumor types.
For Attendees' Attention
Seating is on a first come, first served basis.