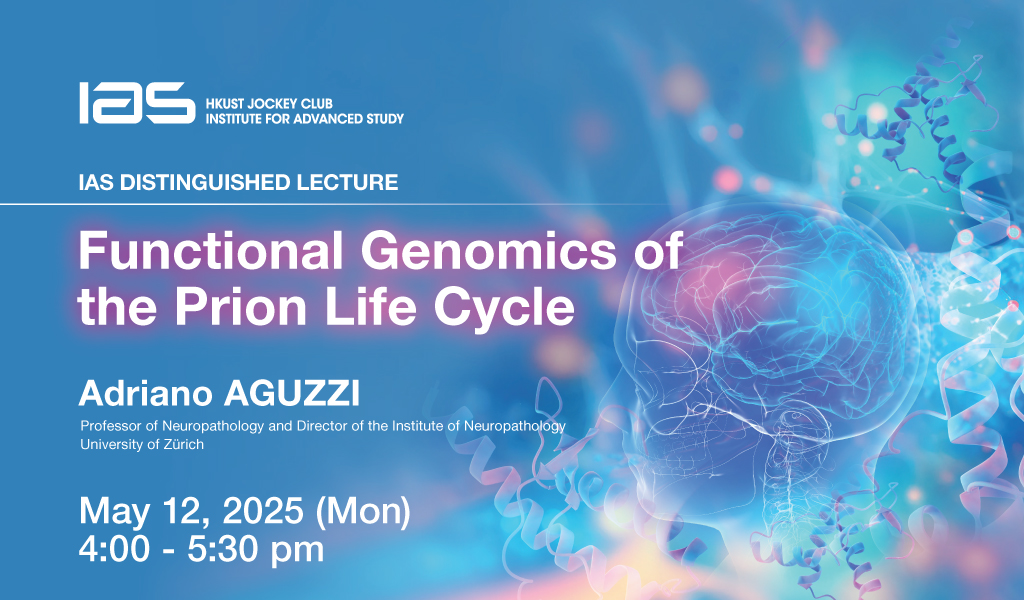
Functional Genomics of the Prion Life Cycle
Abstract
The speaker’s lab uses large-scale genetic perturbations to identify factors modifying discrete steps of the prion life cycle. Because most phenotypes relevant to prion biology require complex biochemical assays and are not selectable with surface markers, they resort to arrayed genetic screens in which the activation or ablation of each gene is tested individually. To achieve this, they invented massively parallel plasmid-cloning methodologies to construct arrayed libraries for the genome-wide ablation, activation and epigenetic silencing of human genes (42’378 discrete lentiviral vectors). In an arrayed genome-wide CRISPR activation screen, they identified 80 and 451 up- and downregulators of PrPC expression, respectively. 45 of the 50 strongest modifiers were confirmed in secondary assays. Many of these genes regulated PRNP transcription despite not being canonical transcription factors, and some affected PRNP transcription antithetically to PrPC protein expression, suggesting posttranscriptional regulation. Other PrPC modifiers impinged on lysosomal degradation, cholesterol metabolism, and mitochondrial function. Surprisingly, the strongest PrPC upregulators affected extracellular matrix (ECM) organization. PrPC levels correlated with ECM abundance, potentially mediated through mechanosensation of gliotic tissue stiffening in prion diseases. Prion entry into cells is a fundamental step of the prion life cycle, and its modulation may impact the establishment and spread of prion infections. A genome-wide CRISPR activation screen for modifiers of prion internalization yielded both expected modulators and novel candidates. Surprisingly, the Bone Morphogenetic Protein (BMP) pathway stood out as the most enriched pathway. Most members of the BMP signal-transduction chain (ligands, receptors, transcription factors) lit up in their screens, with activators and inhibitors leading to increased and decreased prion uptake, respectively. With a synthetic-lethality screen they discovered that ciliogenesis is a crucial contributor to Prion toxicity. They also identified Heterogenous Nuclear Ribonucleoprotein K (hnRNPK) as a factor limiting prion replication. Little is known about its function other than it is essential to cell survival. Using a synthetic-viability CRISPR ablation screen, they found that deletion and overexpression of Transcription Factor AP-2γ (TFAP2C) mitigated and enhanced the death of hnRNPK-ablated cells, respectively. hnRNPK ablation suppressed genes related to lipid and glucose metabolism and enhanced catabolism by modulating mTOR and AMPK, whereas TFAP2C overexpression promoted anabolism. TFAP2C overexpression reduced prion propagation in wild-type cells and neutralized prion accumulation in hnRNPK-suppressed cells. Hence, TFAP2C and hnRNPK are genetic interactors controlling cell metabolism, bioenergy and prion propagation. The speaker and his research team are now expanding their approach to more prion-related phenomena, including synuclein phosphorylation after exposure to synthetic preformed synuclein fibrils. Their results showcase the awesome power of unbiased genetic perturbations for the study of neurodegenerative diseases and may enable the identification of novel therapeutically actionable targets.
About the Speaker
Prof. Adriano AGUZZI earned his MD from the University of Freiburg Medical School in 1986. Following postdoctoral studies in Vienna, he obtained the venia legendi in Neuropathology at the University of Zürich in 1993. He is the Founder and Director of the Swiss National Reference Center for Prion Diseases. He is currently a Full Professor of Neuropathology with Tenure and the Director of the Institute of Neuropathology at the University of Zürich. He is also a Joint Professor of the Faculties of Medicine and Natural Sciences there.
Prof. Aguzzi’s research focuses on studying the molecular basis of prion diseases (rare progressive neurodegenerative disorders), combining transgenetics with molecular and immunological techniques. His pioneering work uncovered the crucial steps in the pathogenesis of the disease, revealed the cells and molecules involved in prion neuroinvasion, and elucidated the mechanisms leading to brain damage in these diseases. Currently, he is investigating the fundamental mechanisms of neurodegeneration. He serves on the editorial board of Science and is the Editor-in-Chief of the Swiss Medical Weekly. He also serves on the scientific advisory board of philanthropic foundations and biomedical companies.
Among other honors, Prof. Aguzzi has won the Gold Medal of the European Molecular Biology Organization (1998), the Ernst Jung Prize in Medicine (1999), the Robert Koch Award (2003), the Baillet Latour Health Prize (2017) and the NOMIS Distinguished Scientist Award (2019). He has also been awarded two ERC Advanced Grants from the European Research Council.
For Attendees' Attention
Seating is on a first come, first served basis.